 QT Vascular CEO, Dr Eitan Konstantino, has a 1.73% stake in the company. The major shareholder is venture capital fund, Three Arch Partners, with a 20.36% stake.DESPITE BEING an early-stage company as well as loss-making, QT Vascular has gained market acceptance, if its stock price performance is anything to go by.
QT Vascular CEO, Dr Eitan Konstantino, has a 1.73% stake in the company. The major shareholder is venture capital fund, Three Arch Partners, with a 20.36% stake.DESPITE BEING an early-stage company as well as loss-making, QT Vascular has gained market acceptance, if its stock price performance is anything to go by.
The company, which has developed breakthrough products for treating blocked arteries, was listed on Catalist on 29 April this year, raising S$55 million through a placement of 196.429 million shares at 28 cents each.
Resilient stock price
Until the recent market weakness, its stock price has held up well above 40 cents after it broke this barrier within one month of listing.
Post-listing, it announced that 2QFY2014 net loss increased by 11.9% year-on-year to US$13.5 million.
What's more important is: Revenue had increased by a whopping 213.5% to US$3.2 million mainly due to an increase in the sale of its Chocolate® PTA Balloon Catheter (“Chocolate PTA”) after Johnson & Johnson unit Cordis started distributing its products.
And 2QFY2014 was the first quarter its posted a gross profit: US$181,000.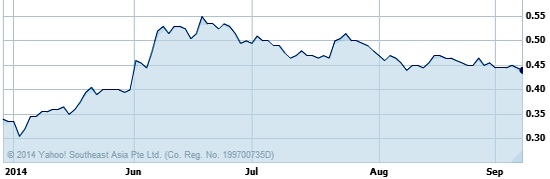 QT Vascular closed at 44 cents on 9 September 2014, more than 50% above its listing price. Its stock price was not much affected after it posted on 14 August that its net loss had widened year-on-year. Chart: Yahoo Finance
QT Vascular closed at 44 cents on 9 September 2014, more than 50% above its listing price. Its stock price was not much affected after it posted on 14 August that its net loss had widened year-on-year. Chart: Yahoo Finance
M&A business model
Analysts are upbeat on the stock: UOB Kayhian maintained its 'Buy' call with a target price of 60 cents while CIMB reiterated its 'Add' rating with a target price of 73 cents.
The reason for the analysts' optimism was the structure of the medical device industry.
Large players focus on capturing market share through greater distribution scale.
On the other hand, smaller companies like QT Vascular focus on R&D of new technologies with the intention of selling out subsequently to larger peers.
A recent example was the acquisition of IDEV Technologies (which makes SUPERA stents) by Abbott for US$310 million at a price to sales multiple of 10.3x.
QT Vascular already has a suitor: Other than being a distributor, Johnson and Johnson is also a pre-listing investor through its venture capital subsidiary, J&J Development Corporation.
That is why the QT Vascular management measures success by the commercial acceptance of its products, rather than the bottom line.
Other than one-off IPO expenses, its focus on R&D innovation was one main reason for its 2QFY2014 net loss, with the R&D expense ratio at a high 58%.
CIMB analyst Gary Ng expects this ratio to remain as high as 45% as the group embarks on its next wave of product development.
This business model was convincing enough for EDB subsidiary Biomedical Sciences Investment Fund (BMSIF) to provide seed funding for QT Vascular.
BMSIF still holds a 8.35% stake in QT Vascular.
Commercialization momentum
Founded by a brilliant Israeli Dr Eitan Konstantino, 46, the high-tech company specializes in making devices used in surgeries for opening blocked arteries in both the heart and legs.
Its products are currently distributed in the US, Europe, Japan and China.
| QT Vascular's Glider™ PTCA Balloon Catheter is the only torqueable angioplasty balloon catheter. Its ability to rotate enables it to cross through tight lesions and stent struts which challenge other types of balloons. This unique design provides physicians with a powerful tool in managing the treatment of a broad range of complex lesions. Video from company |
In late August, its wholly-owned US subsidiary, TriReme Medical LLC, secured a Japanese patent for technology used in surgical procedures for coronary interventions.
Significant elements of this technology are used in the Group’s Glider PTCA balloon catheter.
Glider has demonstrated an ability to cross in cases where other balloons failed and were not able to complete the procedure (see video above).
Exciting times ahead
Other than Cordis Corporation, QT Vascular also has distribution agreements with Century Medical for the sale of its products in Japan and with Weihai Weigao Medical Devices in the PRC.
Sales of its Glider products in Asia in 2HFY2014 are expected to have positive revenue contribution as orders from China and Japan are scheduled for shipment by 4QFY2014.
With the distribution agreements helping to lower operating expenses, Mr Ng of CIMB believes the company may turn around next year.
Its flagship product, Chocolate PTA, has received regulatory approvals and is in commercial launch in numerous countries, including Japan, the United States, and Europe.
Its new product, Silk PTA, is currently at the design feasibility stage with CE mark submission targeted for 1QFY2015.






